The Challenge
Every year, approximately 5 million children die before their fifth birthday and approximately 300,000 mothers die due to pregnancy- or childbirth-related causes. Interventions exist that can prevent more than half of these deaths. However, availability of maternal, newborn and child health (MNCH) products remains low (~45% on average) in Sub-Saharan Africa (SSA), where the majority of global under-five and maternal mortality occurs. In order to achieve the UN Sustainable Development Goals to end preventable maternal, newborn and child deaths by 2030, increasing access to life-saving MNCH medicines is essential.
The Opportunity
Historically, MNCH products have not benefited from the kinds of coordinated market shaping interventions that have been more commonplace in other essential health commodity markets, such as vaccines and HIV/AIDS products. These market shaping solutions are critical to rationalize products, reduce costs, ensure high quality, increase efficiencies and improve commodity availability. With support from the Gates Foundation, Results for Development (R4D) aims to diagnose MNCH market challenges at global, regional and country levels and support governments to design and implement solutions.
Our Work
Phase 1 (2019-2021) Initial MNCH market assessment: “What’s inhibiting access to MNCH products?”
Through collaborations with procurement agencies, national drug regulatory authorities, ministries of health, implementing partners, development partners and other key MNCH stakeholders, R4D systematically analyzed financing, procurement, pricing, quantification and regulatory data across five sub-Saharan African geographies – Ethiopia, Kenya, Tanzania, Uganda and the state of Kano in Nigeria – which in aggregate, procure large volumes of MNCH products and are all Global Financing Facility (GFF) countries.
The market diagnostic confirmed MNCH commodity markets are primarily government-led across LMICs, as shown in the graphic below.
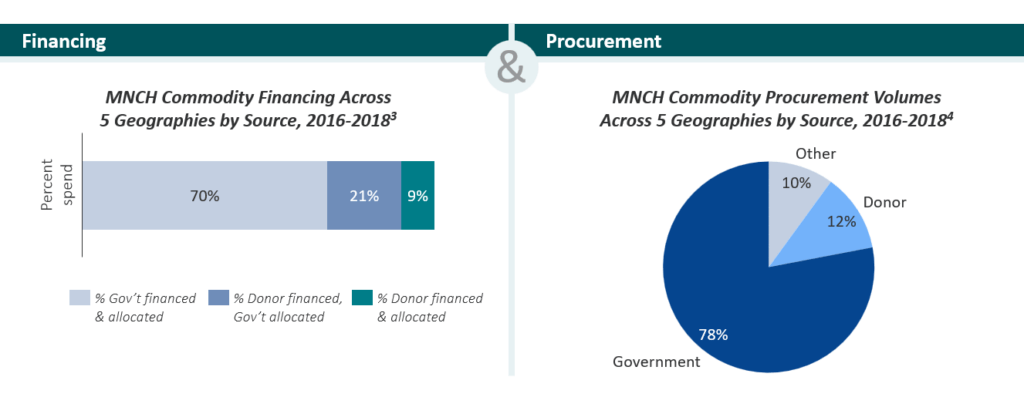
However, MNCH market challenges are inhibiting scale-up of these life-saving commodities. The diagnostic identified the following key challenges:
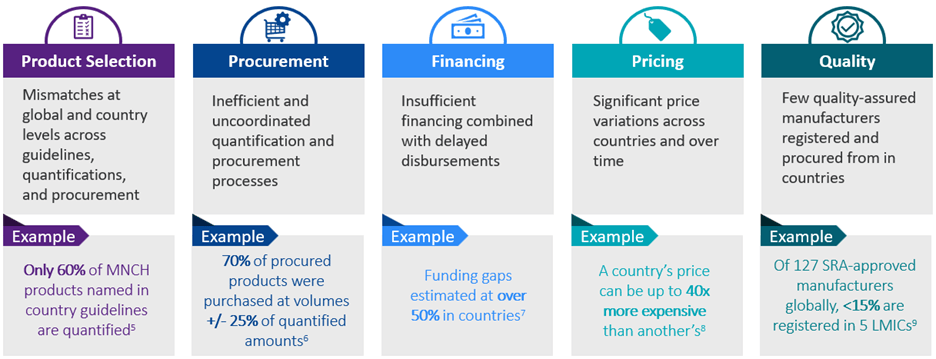
Phase 2 (2021-2022) Strategy development: “What are the right next steps to address MNCH market challenges?”
R4D then developed solutions to the market challenges identified through its comprehensive assessment. Solutions that harmonize the market were co-created with a range of relevant market actors at global, regional, and country levels. These solutions will enable decision-makers to make well-informed strategic decisions regarding how best to catalytically implement changes to improve access for life-saving MNCH medicines.
Implementation in Ethiopia (2022-present) Addressing key MNCH market challenges
Building on findings from the MNCH market assessment and strategy development, R4D is currently working with the Government of Ethiopia to:
- Widen the narrow MNCH supply base
- Support regional harmonization efforts
- Generate evidence of access barriers at last mile distribution
- Design sustainable financing sources
2024 MNCH Market Outlook (2024-present) Updating MNCH market understanding post-COVID
To support partners and governments to operate with the most accurate information, R4D is updating its 2020 MNCH market assessment – “2024 MNCH Market Outlook”. These findings will serve to (1) capture key market changes following the COVID-19 pandemic, and (2) serve as a baseline understanding of the MNCH market to measure impact of market shaping strategies thus ensuring achievement of the Sustainable Development Goals to end preventable maternal, newborn and child deaths by 2030.
The 2024 MNCH Market Outlook will seek to understand current access and market challenges for twelve MNCH products across Ethiopia, Kenya, and 10 states in Nigeria (Bauchi, Borno, Gombe, Kaduna, Kano, Lagos, Nasarrawa, Niger, Sokoto, Yobe). Where opportunistic, the assessment will include additional MNCH commodities identified as national priorities by country governments during the R4D-conducted 2020 MNCH market assessment.














