Historically, the introduction and scale-up of lifesaving health products in low- and middle-income countries has been led by donors and external partners. Results for Development is supporting the Government of Ethiopia to shift the paradigm.
The Challenge
In Ethiopia critical health commodities and products are not always available to people who need them. This is mainly due to funding gaps, inefficiencies in resource mobilization, allocation and utilization, high forecasting error, long procurement lead time and high wastage rates.
Improving access to high quality and affordable essential health and nutrition products is a strategic priority of the Government of Ethiopia. Globally, market shaping approaches have been successfully deployed to increase access to critical medicines; but in Ethiopia, as in most low- and middle-income countries, this is generally done on a product-by-product basis and is often led by and reliant on international partners. As a result, the government is continually dependent on these partners to play a market manager role to support entry of new products into the Ethiopian market.
The Opportunity
The Ethiopian Pharmaceutical Supply Service (EPSS) — the government institution that acts as a supply chain leader in the public health system — has achieved notable improvements in supply chain performance over the last ten years. However, critical gaps remain that impede full access to health and nutrition products. And reliance on donor funding and partner expertise remains high.
To address this, R4D has worked with the Ethiopian Pharmaceutical Supply Service to support the Quantification and Market Shaping Directorate (QMSD) to play a lead role and serve as the market manager, addressing key market barriers, and ultimately increasing the availability of high-quality and affordable health and nutrition products in the public health system.
Our Work
Since 2019, the R4D-led Ethiopia Market Shaping Capacity Improvement Program (EMSCIP) project has partnered with the Ethiopian Pharmaceutical Supply Service to support the development and capacity of QMSD to play the market manager role. In the first phase of the project, R4D focused on the following:
Co-creating evidence: R4D and the Ethiopian Pharmaceutical Supply Service worked together to generate critical evidence for improved decision-making.
- R4D and the Ethiopian Pharmaceutical Supply Service conducted a comprehensive market shortcomings assessment in 2020 to better understand critical market shortcomings and inform the market shaping strategy, actions, and capacity building. The assessment identified critical barriers across financing, pharmaceutical procurement list, product registration, forecasting, tender and contract management processes. Based on the findings of the assessment, key market interventions were co-developed and implemented. When the assessment was conducted again in 2023, progress was shown in financing for health commodities, procurement processes, the pharmaceutical procurement list, supply base (product registration), wastage rate and forecast accuracy as summarized below:
- Total budget allocated to Revolving Drug Fund (RDF) increased from 4.8 billion ETB (~$86M USD) in 2020 to 9 billion ETB (~$160M USD)
- EPSS credit sales collection performance increased from 71% in 2020 to 81% in 2023
- Procurement lead time reduced from 252 days in 2020 to 194 days in 2023
- Purchase Order (PO) approval reduced from 60 days in 2020 to 10 days in 2023
- EPSS pharmaceutical procurement list rationalization from 1,373 products in 2022 rationalized to 683 products in 2023 through optimized product selection criteria
- The number of products with adequate registered suppliers in country (i.e. >=5 suppliers) increased from 21% in 2020 to 29% in 20203
- Wastage rate reduced from 6% in 2020 to 0.1 % in 2023
- EPSS forecasting accuracy for RDF increased from 35% in 2020 to 60% in 2023
Ensuring clear goals, strategies and tools: R4D has supported the Ethiopian Pharmaceutical Supply Service to codify a strategic vision for market shaping and to develop an organizational blueprint for QMSD.
- R4D supported the Ethiopian Pharmaceutical Supply to develop its first ever comprehensive market shaping strategy (2023-2025).
- R4D has worked with the QMSD team to develop many important market shaping tools and guides.
Building market shaping expertise and capacity: R4D has provided catalytic support to build and strengthen the capacities of the Ethiopian Pharmaceutical Supply Service leadership and staff, particularly within QMSD, with the ultimate goal of supporting QMSD’s ability to serve in the market manager role.

- R4D and the Ethiopian Pharmaceutical Supply Service conducted a baseline market shaping competency assessment in 2021 to identify key capacity building needs of staff.
- Using findings from the baseline assessment, R4D developed market shaping training materials, including a facilitator’s guide and a trainee’s manual. And in July 2023, the Ethiopian Ministry of Health approved the training as part of the government’s national In-Service Trainings (IST).
- A total of 112 central and hub staff completed the standard market shaping courses. And of the 112 trainees who completed the training, 106 scored above the minimum acceptable score and were certified. When assessed in 2023, QMSD’s market shaping competency had improved across all four target areas.
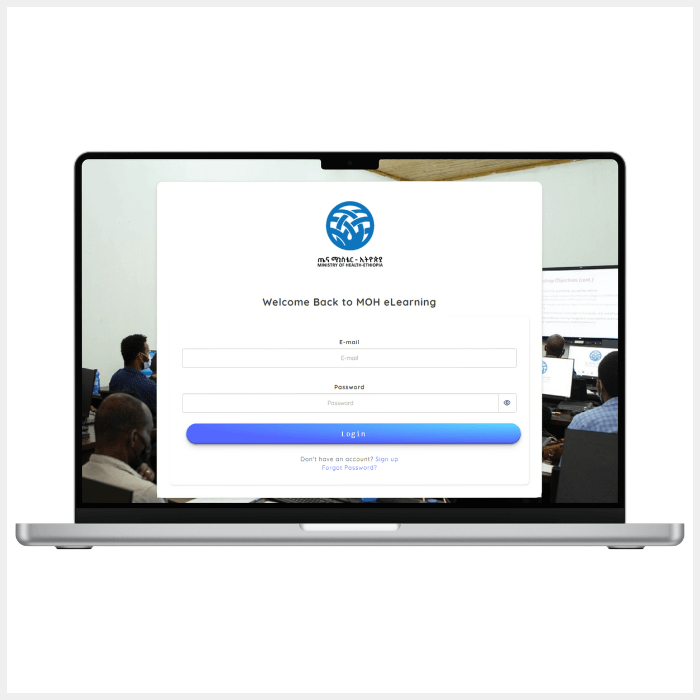
- In 2025, the MOH and EPSS, with support from R4D, launched the Basic Pharmaceutical Market Shaping e-Learning Course. This free, self-paced course, available on the MOH website, introduces practical tools for identifying and addressing market shortcomings. It is designed for health, supply chain, and market shaping professionals who want to strengthen their skills in optimizing pharmaceutical procurement and supply strategies. The course is accredited and offers continuing education units, as well as a certificate upon completion. This represents an important step toward strengthening the quality, equity, accessibility, and sustainability of market-shaping capacity-building efforts in Ethiopia.
Building on its initial success, R4D launched a three-year second phase of the program with nearly $3.4 million in funding from the Bill & Melinda Gates Foundation. Phase two will focus on five key areas:
- Improve institutionalization of market shaping, including coaching for QMSD staff, improve coordination and clarify market manager roles across the Ethiopian Pharmaceutical Supply Service and other government agencies.
- Develop a sustainable financing strategy for commodities, including strengthening the investment case for domestic commodity financing and building consensus on pathways to sustainable commodity financing.
- Address last mile bottlenecks to increase commodity availability by gathering evidence and increasing the knowledge base on challenges and providing strategic recommendations on how to best address them.
- Ensure critical commodities can be sourced by establishing a sourcing strategy, particularly for products that are not included in the Ethiopian Pharmaceutical Supply Service Pharmaceutical Procurement List (PPL).
- Strengthen the National Emergency Supply Chain System by engaging stakeholders to review challenges and identify priority actions, supporting the rollout of a regional emergency supply system management forum, and training health facility staff in emergency supply system management.
Photo credit: R4D